
Get patients started on Mounjaro1
The Mounjaro experience, designed for patients like Julia1: not to A1C goal on metformin, struggling to lose excess weight despite her efforts with diet and exercise

Actor portrayal of an adult woman with type 2 diabetes
Ready to prescribe Mounjaro?
How to Prescribe Mounjaro
Help get patients started on Mounjaro1
Start

Provide patients with a
1-month* 2.5-mg starting
dose prescription
Prescribe
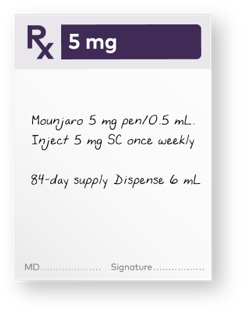
After 4 weeks on the 2.5 mg dose, provide patients with a 1-month* or 3-month† 5.0-mg prescription
Activate

Help your eligible patients save on Mounjaro
For eligible, commercially insured patients with Mounjaro coverage. Governmental beneficiaries excluded, terms and conditions apply
Image depicting how to prescribe Mounjaro. Starting Dose: provide patients with a 1-month 2.5-mg starting dose. Prescribe: After 4 weeks on the 2.5 mg dose, provide patients with a 1-month or 3-month 5 mg prescription.* 2.5 mg prescription. Access: See HCP formulary access and top plans by clicking LEARN MORE. Activate: Help your patients save on Mounjaro. For eligible, commercially insured patients with Mounjaro coverage. Governmental beneficiaries excluded, terms and conditions apply.
Prior authorizations are common for the incretin class. Get tips for Prior Authorization Submission Process.
*One month is defined as 28 days and 4 pens.
†Three months is defined as 84 days and up to 12 pens.
The 2.5-mg dose is for treatment initiation and is not intended for glycemic control.
See Savings Card Terms and Conditions
2.5-mg dose NDC: 0002-1506-80; 5-mg dose NDC: 0002-1495-80l
How to Dose Mounjaro
Multiple doses for customizable glycemic control1‡
START THE EXPERIENCE
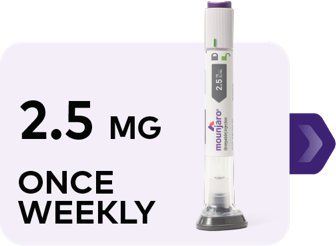
Starting dose (for 4 weeks)
MONTH 1
CONTINUE THE EXPERIENCE

For at least 4 weeks
MONTH 2
IF ADDITIONAL GLYCEMIC CONTROL IS NEEDED

For at least 4 weeks

For at least 4 weeks

For at least 4 weeks
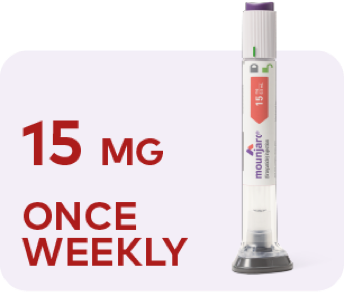
Maximum dose
Image depicting escalation of Mounjaro. Starting dose is 2.5 mg once weekly for 4 weeks. Continue to 5 mg once weekly for at least 4 weeks. If additional glycemic control is needed, dose can be increased to 7.5 mg once weekly for at least 4 weeks, then 10 mg once weekly for at least 4 weeks, then 12.5 mg once weekly for at least 4 weeks, and then 15 mg once weekly as a maximum dose.
If additional glycemic control is needed, you can continue to increase the dose by 2.5-mg increments after at least 4 weeks on the current dose. The maximum
dose is 15 mg once weekly.
The 2.5-mg dose is for treatment initiation and is not intended for glycemic control.†
*Consider patient history and monitor for tolerability and side effects.
‡Across the five phase 3 SURPASS studies, mean reductions in A1C with Mounjaro ranged from 1.8% to 2.1% for the 5-mg dose, 1.7% to 2.4% for
the 10-mg dose, and 1.7% to 2.4% for the 15-mg dose; and for comparators, 0.1% and 0.9% for placebo, 1.9% for Ozempic® 1 mg, 1.3% for Tresiba®, and 1.4% for
insulin glargine. p<0.05 for superiority vs. study comparators, adjusted for multiplicity.1
Setting Patient Expectations
Tips and resources for you and your staff
Resources for your patients
Digital Starter Kit
Tell your patients to text MJ to 85099 to download a free kit to help them get started on Mounjaro.
The kit provides tips on starting Mounjaro, what to expect, reminder options and additional resources.
HCP and Patient Testimonials
Hear from adult patients and their health care provider on why Mounjaro was an appropriate choice of T2D therapy for them.
[AVO]
Mounjaro is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Mounjaro has not been studied in patients with a history of pancreatitis. Mounjaro is not indicated for use in patients with type 1 diabetes mellitus.
WARNING: RISK OF THYROID C-CELL TUMORS
In both male and female rats, tirzepatide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Mounjaro causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined.
Mounjaro is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (for example, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro.
I'm Bob. And I'm Nancy. We've been married for 43 years.
Once we were diagnosed with type 2 diabetes, we knew that we needed to get our A1C in check. And when we started Mounjaro, it's been basically a team effort, both of us traveling this road together.
I'm Stacy Brown. I'm a nurse practitioner in Tennessee, and I've been practicing for 27 years with an emphasis on endocrinology.
I first heard about Mounjaro before it was even “Mounjaro,” in the early phases, through articles and through journals.
After looking at Bob and Nancy's medical histories and taking into consideration safety factors, I determined that both of them were good candidates for Mounjaro for the treatment of their type 2 diabetes, although they were at different stages of their type 2 diabetes.
Well, when it was recommended that I start, that I had the prescription filled at our local pharmacy and started it the very next day.
My wife and I, we take our shots on Thursday mornings.
On Wednesday night, we put a little paper note up on the bathroom mirror, so when we get up Thursday morning, it's a reminder for us, kind of a normal routine week after week now, every Thursday morning.
When patients like Bob and Nancy come to see me and they've had such success with their A1C results, of course I'm happy as a clinician. And you also see that that success begets success, and you see that they're very motivated to keep going even with their lifestyle changes.
And not only that, they've both experienced weight loss.
Now that our A1C is under control, we're more motivated to do things together, plan our meals together; we plan our activities together and our exercises together.
Also, we have a granddaughter. We feel a whole lot better about doing things with her.
The effectiveness of Mounjaro as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes was established in five phase 3 clinical studies.
In SURPASS-1 through -5, patients were randomized to receive Mounjaro 5, 10, or 15 milligrams, placebo, or active comparator.
Background glucose-lowering therapy in the SURPASS studies included various combinations of metformin, SGLT2 inhibitors, sulfonylurea, titrated insulin glargine, or no background glucose-lowering therapy at all.
Comparator treatments in the SURPASS studies included placebo, Ozempic 1 milligram, titrated Tresiba, and titrated insulin glargine.
The primary endpoint across the SURPASS studies was the mean change in A1C from baseline at 40 or 52 weeks.
Mounjaro demonstrated superior A1C reductions across the SURPASS clinical studies.
In the SURPASS-1 and -5 placebo-controlled studies, mean reductions in A1C from baseline to week 40 for the Mounjaro 5-, 10-, and 15-milligram groups ranged from 1.7% to 2.4% compared with 0.1% to 0.9% for the placebo groups.
Adverse reactions in the pool of placebo-controlled studies reported in at least 5% of patients taking Mounjaro included nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain.
Treatment discontinuation due to gastrointestinal-related adverse events ranged from 3.0% to 6.6% for the Mounjaro 5-, 10-, and 15-milligram groups compared to 0.4% for the placebo groups.
Mounjaro is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with known serious hypersensitivity to tirzepatide or any of the excipients in Mounjaro. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Mounjaro.
Risk of Thyroid C-cell Tumors: Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (for example, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists. Pancreatitis has been reported in Mounjaro clinical trials. Mounjaro has not been studied in patients with a prior history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on Mounjaro. Observe patients for signs and symptoms, including persistent severe abdominal pain sometimes radiating to the back, which may or may not be accompanied by vomiting. If pancreatitis is suspected, discontinue Mounjaro and initiate appropriate management.
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue (for example, sulfonylurea) or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Hypersensitivity Reactions: Serious hypersensitivity reactions (for example, anaphylaxis, angioedema) have been reported in patients treated with Mounjaro. If hypersensitivity reactions occur, discontinue use of Mounjaro; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous serious hypersensitivity to Mounjaro. Use caution in patients with a history of angioedema or anaphylaxis with a GLP-1 receptor agonist because it is unknown if such patients will be predisposed to these reactions with Mounjaro.
Acute Kidney Injury: Mounjaro has been associated with gastrointestinal adverse reactions, which include nausea, vomiting, and diarrhea. These events may lead to dehydration, which if severe could cause acute kidney injury. In patients treated with GLP-1 receptor agonists, there have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, sometimes requiring hemodialysis. Some of these events have been reported in patients without known underlying renal disease. A majority of reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of Mounjaro in patients with renal impairment reporting severe adverse gastrointestinal reactions.
Severe Gastrointestinal Disease: Use of Mounjaro has been associated with gastrointestinal adverse reactions, sometimes severe. Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
Diabetic Retinopathy Complications in Patients with a History of Diabetic Retinopathy: Rapid improvement in glucose control has been associated with a temporary worsening of diabetic retinopathy. Mounjaro has not been studied in patients with non-proliferative diabetic retinopathy requiring acute therapy, proliferative diabetic retinopathy, or diabetic macular edema. Patients with a history of diabetic retinopathy should be monitored for progression of diabetic retinopathy.
Acute Gallbladder Disease: In clinical trials, acute gallbladder disease was reported by 0.6% of Mounjaro-treated patients and 0% of placebo-treated patients. If cholelithiasis is suspected, gallbladder diagnostic studies and appropriate clinical follow-up are indicated.
The most common adverse reactions reported in ≥5% of Mounjaro-treated patients in placebo-controlled trials were nausea, diarrhea, decreased appetite, vomiting, constipation, dyspepsia, and abdominal pain.
Drug Interactions: When initiating Mounjaro, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia. Mounjaro delays gastric emptying, and thereby has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised.
Pregnancy: Limited data on Mounjaro use in pregnant women are available to inform on drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Based on animal reproduction studies, there may be risks to the fetus from exposure to tirzepatide. Use only if potential benefit justifies the potential risk to the fetus.
Lactation: There are no data on the presence of tirzepatide in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for Mounjaro and any potential adverse effects on the breastfed infant from Mounjaro or from the underlying maternal condition.
Females of Reproductive Potential: Advise females using oral hormonal contraceptives to switch to a non-oral contraceptive method, or add a barrier method of contraception for 4 weeks after initiation and for 4 weeks after each dose escalation.
Pediatric Use: Safety and effectiveness of Mounjaro have not been established and use is not recommended in patients less than 18 years of age.

Discover Lilly Play
Watch videos about Mounjaro and how to help your patients get started.
Prescribing in your EHR System
Help patients get from initiation to a therapeutic dose of Mounjaro
Select Important Safety Information:
Risk of Thyroid C-cell Tumors: Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
References:
- Mounjaro. Instructions for Use. Lilly USA, LLC.
- Mounjaro. Prescribing Information. Lilly USA, LLC.
- Maceira E, Lesar TS, Smith HS. Medication related nausea and vomiting in palliative medicine. Ann Palliat Med. 2012;1(2):161-176.
- Kruger DF, Bode B, Spollett GR. Understanding GLP-1 analogs and enhancing patients success. Diabetes Educ. 2010;36(Suppl 3):44S-72S.
- Reid TS. Practical use of glucagon-like peptide-1 receptor agonist therapy in primary care. Clin Diabetes. 2013;31(4):148-157

Mounjaro (tirzepatide) is currently only available in a pre-filled single-dose pen manufactured by Lilly. Mounjaro is not commercially available in any other form (e.g., powder for compounding). Products claiming to be compounded Mounjaro (tirzepatide) are not subject to FDA approval and may not have the same safety, quality and efficacy as FDA-approved drugs, and may expose patients to potentially serious health risks.





