Mounjaro works differently than a GLP-1 RA1
Tirzepatide is the first and only approved single molecule that activates GIP and GLP-1 receptors in the body.1
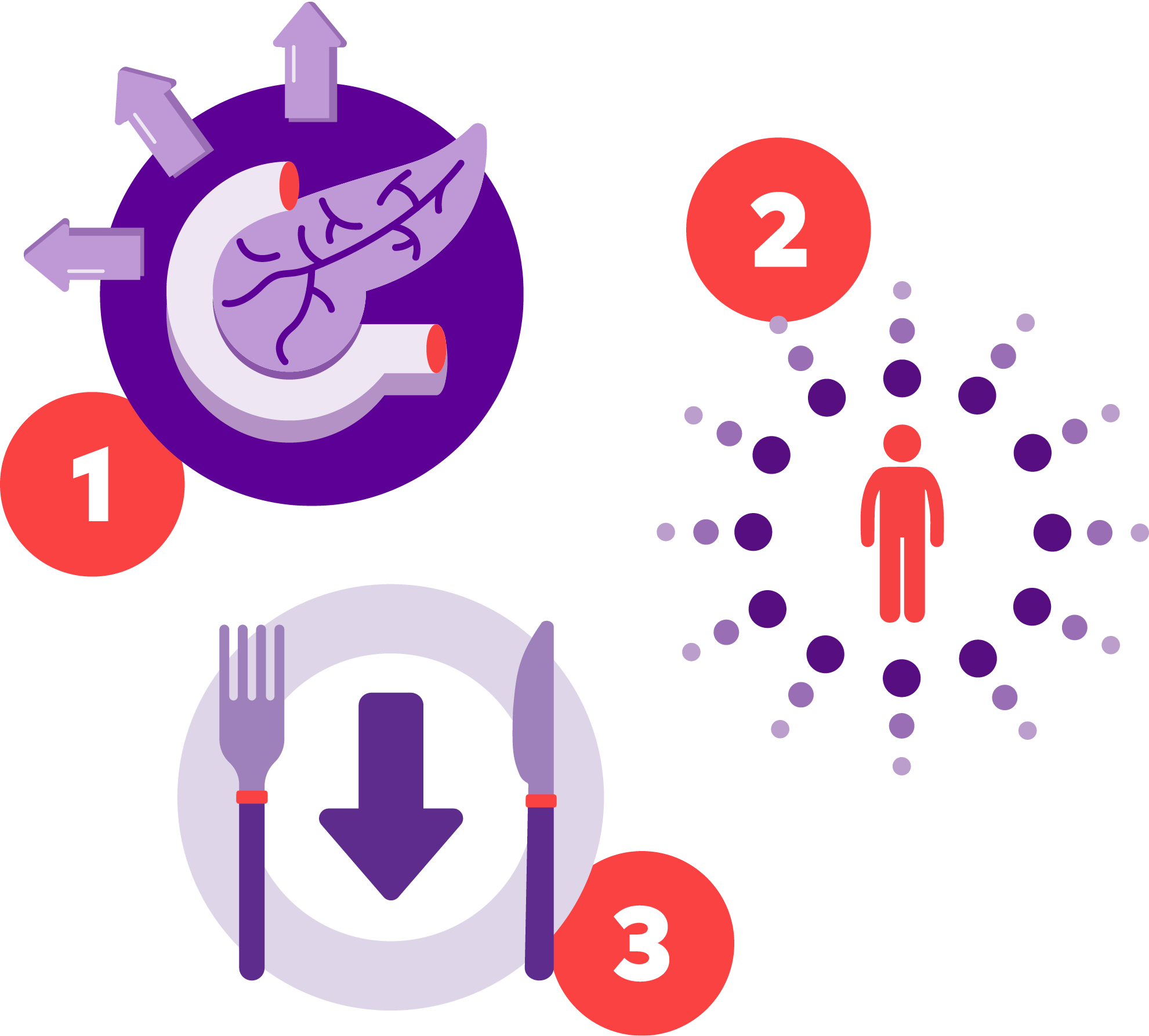
Mounjaro acts in the following ways1:
1. ENHANCES insulin secretion
2. IMPROVES insulin sensitivity
3. DECREASES food intake
Additional actions include delayed gastric emptying* and reduced glucagon levels.
Resulting in lower glucose concentrations in both fasting and postprandial states.
*This effect diminishes over time.
GIP=glucose-dependent insulinotropic polypeptide; GLP-1=glucagon-like peptide-1; RA=receptor agonist; T2D=type 2 diabetes.
Mounjaro MOA video
[AVO]
What is Mounjaro? Mounjaro (tirzepatide), an injectable prescription medicine, is indicated as an adjunt to diet and exercise to improve glycemic control with type 2 diabetes mellitus. Limitations of Use: Mounjaro has not been studied in patients with a history of pancreatitis. Mounjaro is not indicated for use in patients with type 1 diabetes mellitus.
Mounjaro includes a boxed warning related to risk of thyroid C-cell tumors. In both male and female rats, tirzepatide causes dose-dependent and treatment-duration-dependent thyroid C-cell tumors at clinically relevant exposures. It is unknown whether Mounjaro causes thyroid C-cell tumors, including medullary thyroid carcinoma (MTC), in humans as human relevance of tirzepatide-induced rodent thyroid C-cell tumors has not been determined. Mounjaro is contraindicated in patients with a personal or family history of MTC or in patients with Multiple Endocrine Neoplasia syndrome type 2 (MEN 2). Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (for example, a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro.
[VO]
How does Mounjaro bind to GIP and GLP-1 receptors?
The native incretin hormones, GIP and GLP-1, bind to their respective receptors on beta cells in the pancreas to enhance insulin release.
Mounjaro is a single peptide designed from the structure of native GIP and modified to bind to the GIP and GLP-1 receptors.
While the activity of Mounjaro on the GLP-1 receptor is lower than the activity of the native GLP-1 hormone, the activity of Mounjaro on the GIP receptor is similar to that of the native GIP hormone.
Where does Mounjaro act in the body? In individuals with type 2 diabetes, Mounjaro improves glycemic control through several mechanisms.
It directly stimulates insulin secretion by activating GIP and GLP-1 receptors in the pancreas, improves insulin sensitivity and decreases food intake.
Mounjaro also reduces blood glucagon concentrations and slows postprandial glucose absorption by delaying gastric emptying, though the latter effect is largest after the first dose and diminishes with time.
All these actions work together to lower blood glucose concentration in both the fasting and postprandial states.
Why does Mounjaro only need to be given once weekly?
Mounjaro is given as a subcutaneous injection and typically reaches its maximum plasma concentration within 8 to 72 hours.
It was specifically engineered with a C20 fatty diacid portion that allows binding to serum albumin, extending the elimination half-life to approximately 5 days and enabling once-weekly dosing.
Following initiation of the 2.5-milligram dose, the plasma concentration of Mounjaro increases and reaches a steady state. The same pattern holds true when patients who are already receiving Mounjaro are escalated to a higher dose.
How is Mounjaro metabolized and eliminated in the body?
Mounjaro is metabolized by proteolytic cleavage of the peptide backbone, beta oxidation of the C20 fatty diacid moiety, and amide hydrolysis.
Mounjaro has low potential to inhibit or induce CYP enzymes.
Neither renal impairment nor hepatic impairment impact the overall pharmacokinetics of Mounjaro, so no dose adjustment is recommended for people with either of these conditions.
In summary, Mounjaro is a single molecule that activates the GIP and GLP-1 receptors in the body.
Its structure is based on the amino-acid backbone of native GIP, with modifications to allow binding to GIP and GLP-1 receptors. Its half-life has been extended by the addition of a fatty diacid group to allow once-weekly dosing. It has a multifaceted glucose-lowering action that functions by increasing insulin secretion, improving insulin sensitivity, decreasing food intake, lowering glucagon concentrations, and delaying gastric emptying.
Together, these actions have established Mounjaro as the first in a new class of therapy for people with type 2 diabetes.
Reference:
- Mounjaro. Prescribing Information. Lilly USA, LLC.