Reset Your Expectations with Mounjaro1*
Every dose of Mounjaro is superior to Ozempic® (semaglutide) 1 mg in reducing A1C1*
The primary endpoint was mean change in A1C from baseline at 40 weeks.
IN ADULT PATIENTS WITH TYPE 2 DIABETES (T2D) ON METFORMIN
Reductions in A1C from baseline (%)1
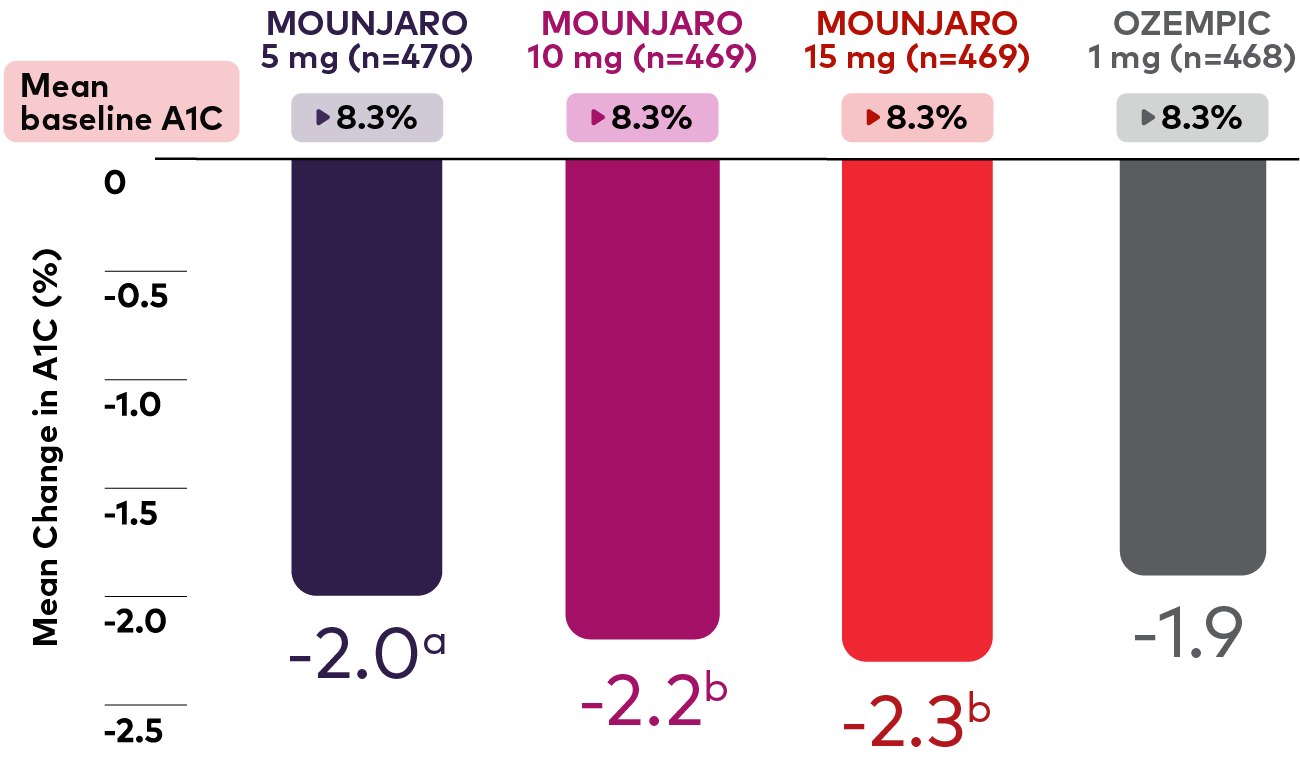
Bar chart depicting A1C change from baseline at 40 weeks. There were 470, 469, 469, and 468 patients in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and each treatment group had a mean baseline A1C of 8.3%. Mean change in A1C at 40 weeks was - 2.0% (p<0.05 for superiority vs. Ozempic, adjusted for multiplicity), -2.2% (p<0.001 for superiority vs. Ozempic, adjusted for multiplicity), -2.3% (p<0.001 for superiority vs. Ozempic, adjusted for multiplicity), and -1.9% for Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg, respectively
Patients reached up to 2.3% A1C reduction with Mounjaro 15 mg1*
*In other studies of glycemic control with a primary endpoint at 40 weeks or 52 weeks, mean reductions in A1C with Mounjaro ranged from 1.8% to 2.1% for the 5-mg dose, 1.7% to 2.4% for the 10-mg dose, and 1.7% to 2.4% for the 15-mg dose; and for comparators, 0.1% and 0.9% for placebo, 1.3% for Tresiba®, and 1.4% for insulin glargine.1
Select Important Safety Information
Mounjaro is contraindicated in patients with a personal or family history of MTC or in patients with MEN 2, and in patients with known serious hypersensitivity to tirzepatide or any of the excipients in Mounjaro. Serious hypersensitivity reactions including anaphylaxis and angioedema have been reported with Mounjaro.

Discover the weight reduction results reported in a clinical trial
Mounjaro is not indicated for weight loss.
Change in weight was a secondary endpoint.
Sustained A1C reductions at every dose1*
The primary endpoint was mean change in A1C from baseline at 40 weeks.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Mean A1C over time from baseline to week 401
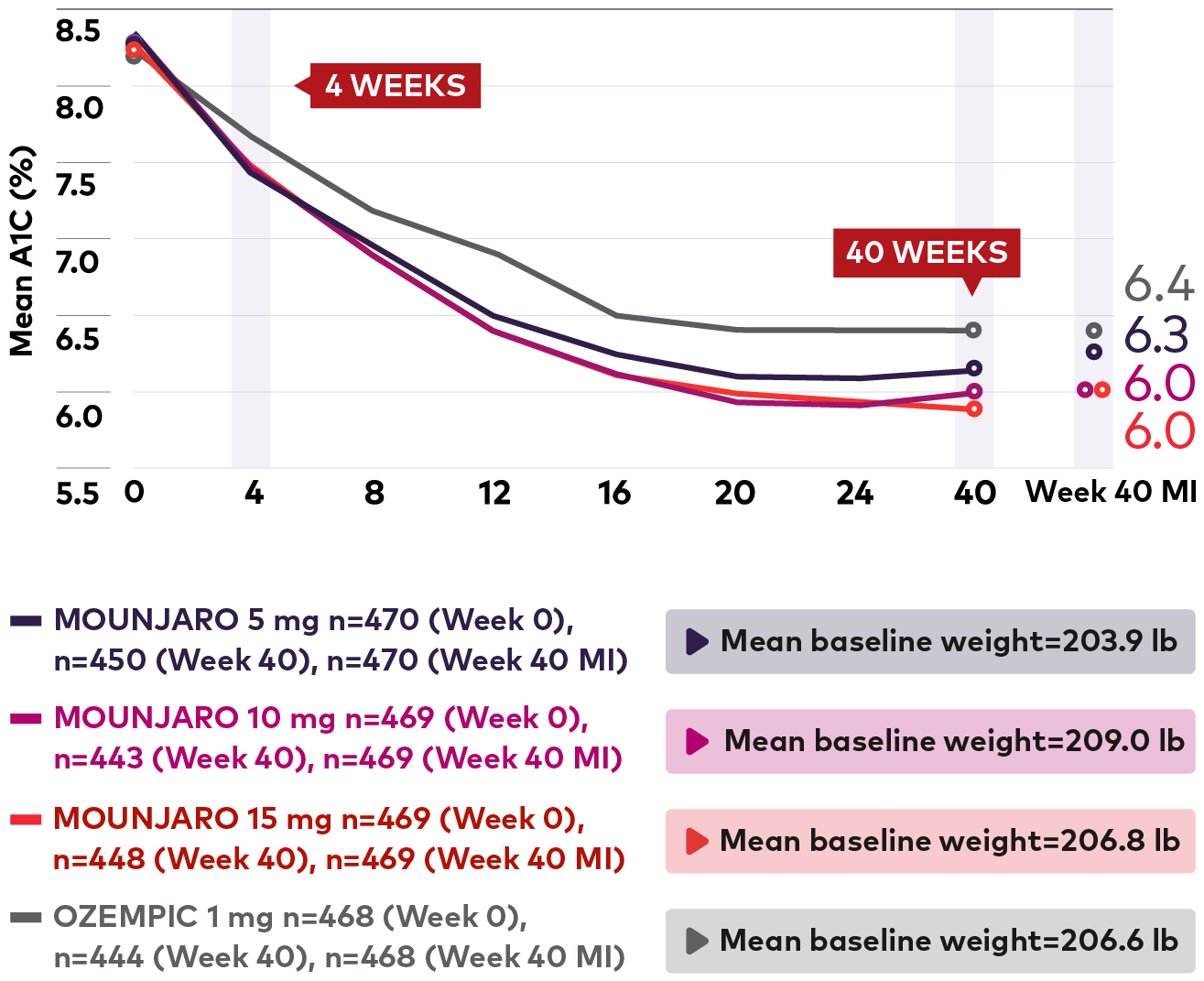
Line graph showing mean A1C over 40 weeks. 470 patients randomized to Mounjaro 5 mg achieved a mean A1C of 6.3% at the end of 40 weeks from a mean baseline A1C of 8.3%. 469 patients randomized to Mounjaro 10 mg achieved a mean A1C of 6.0% at the end of 40 weeks from a mean baseline A1C of 8.3%. 469 patients randomized to Mounjaro 15 mg achieved a mean A1C of 6.0% at the end of 40 weeks from a mean baseline A1C of 8.3%. 468 patients randomized to Ozempic 1 mg achieved a mean A1C of 6.4% at the end of 40 weeks from a mean baseline A1C of 8.3%
Mean A1C reductions were observed starting at week 4 and continued through week 401
*In other studies of glycemic control with a primary endpoint at 40 weeks or 52 weeks, mean reductions in A1C with Mounjaro ranged from 1.8% to 2.1% for the 5-mg dose, 1.7% to 2.4% for the 10-mg dose, and 1.7% to 2.4% for the 15-mg dose; and for comparators, 0.1% and 0.9% for placebo, 1.3% for Tresiba®, and 1.4% for insulin glargine.1
Data represent observed means from week 0 to week 40, and least-squares mean at week 40 MI. mITT population, full analysis set. ANCOVA analysis was performed for change from baseline at week 40 and MMRM analysis was performed for change over time.
ANCOVA=analysis of covariance; MI=multiple imputation; mITT=modified intent-to-treat; MMRM=mixed model for repeated measures.
Select Important Safety Information
Risk of Thyroid C-cell Tumors: Counsel patients regarding the potential risk for MTC with the use of Mounjaro and inform them of symptoms of thyroid tumors (e.g., a mass in the neck, dysphagia, dyspnea, persistent hoarseness). Routine monitoring of serum calcitonin or using thyroid ultrasound is of uncertain value for early detection of MTC in patients treated with Mounjaro. Such monitoring may increase the risk of unnecessary procedures, due to the low test specificity for serum calcitonin and a high background incidence of thyroid disease. Significantly elevated serum calcitonin values may indicate MTC and patients with MTC usually have calcitonin values >50 ng/L. If serum calcitonin is measured and found to be elevated, the patient should be further evaluated. Patients with thyroid nodules noted on physical examination or neck imaging should also be further evaluated.
Significantly more patients achieved the ADA guideline-recommended A1C target of <7%†, ‡ with Mounjaro 10 mg and 15 mg1,2
The primary endpoint was mean change in A1C from baseline at 40 weeks.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Percentage of Patients With A1C <7%1
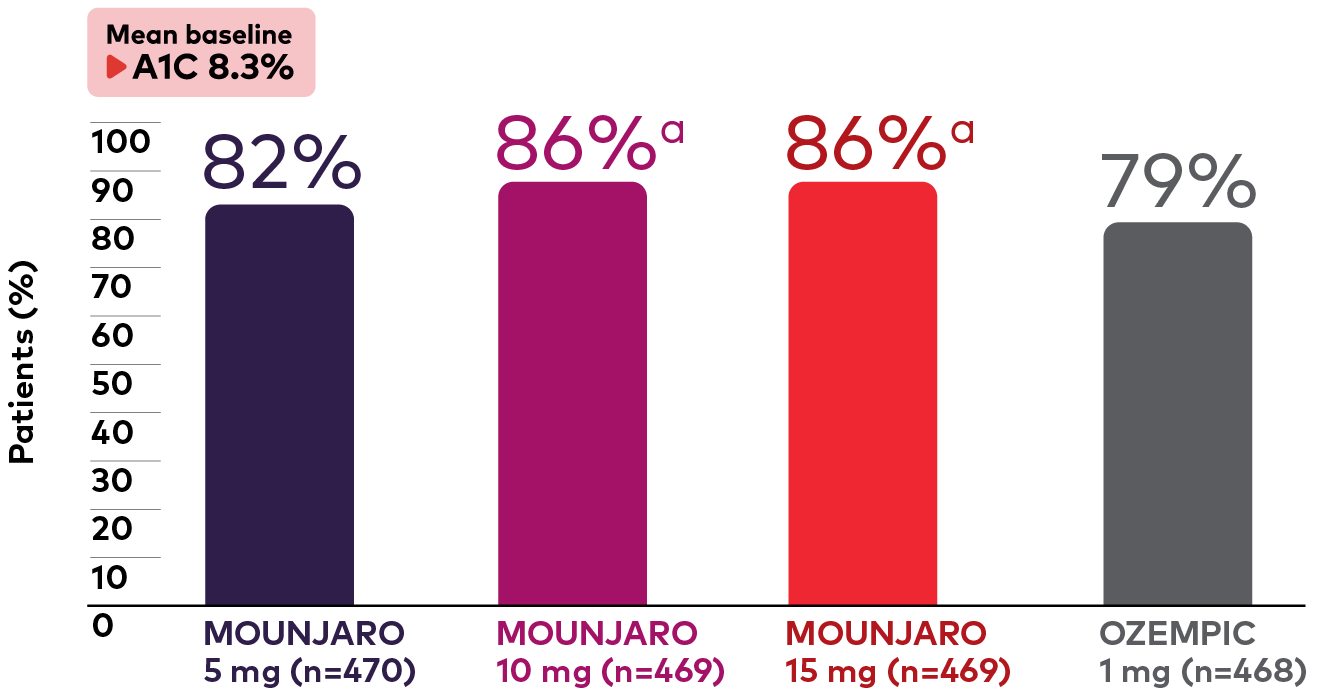
Bar graph showing the percentage of adult patients with type 2 diabetes on metformin in the SURPASS-2 study who had an A1C of less than 7% at 40 weeks. The graph shows Mounjaro vs Ozempic 1 mg. From a mean baseline A1C of 8.3%, 82% (Mounjaro 5 mg, n=470), 86% (Mounjaro 10 mg, n=469), 86% (Mounjaro 15 mg, n=469), and 79% (Ozempic 1 mg, n=468) of patients had an A1C of less than 7%.
†The ADA states that an A1C target of <7% (53 mmol/mol) without significant hypoglycemia is appropriate for many nonpregnant adults.2
‡Key secondary endpoint controlled for type I error. Logistic regression adjusted for baseline value and other stratification factors.
ADA=American Diabetes Association.
Select Important Safety Information
Pancreatitis: Acute pancreatitis, including fatal and non-fatal hemorrhagic or necrotizing pancreatitis, has been observed in patients treated with GLP-1 receptor agonists. Pancreatitis has been reported in Mounjaro clinical trials. Mounjaro has not been studied in patients with a prior history of pancreatitis. It is unknown if patients with a history of pancreatitis are at higher risk for development of pancreatitis on Mounjaro. Observe patients for signs and symptoms including persistent severe abdominal pain sometimes radiating to the back, which may or may not be accompanied by vomiting. If pancreatitis is suspected, discontinue Mounjaro and initiate appropriate management.
Percentage of patients with A1C ≤6.5% and <5.7%1,3
The primary endpoint was mean change in A1C from baseline at 40 weeks.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Percentage of Patients With A1C ≤6.5% at 40 Weeks1,3§
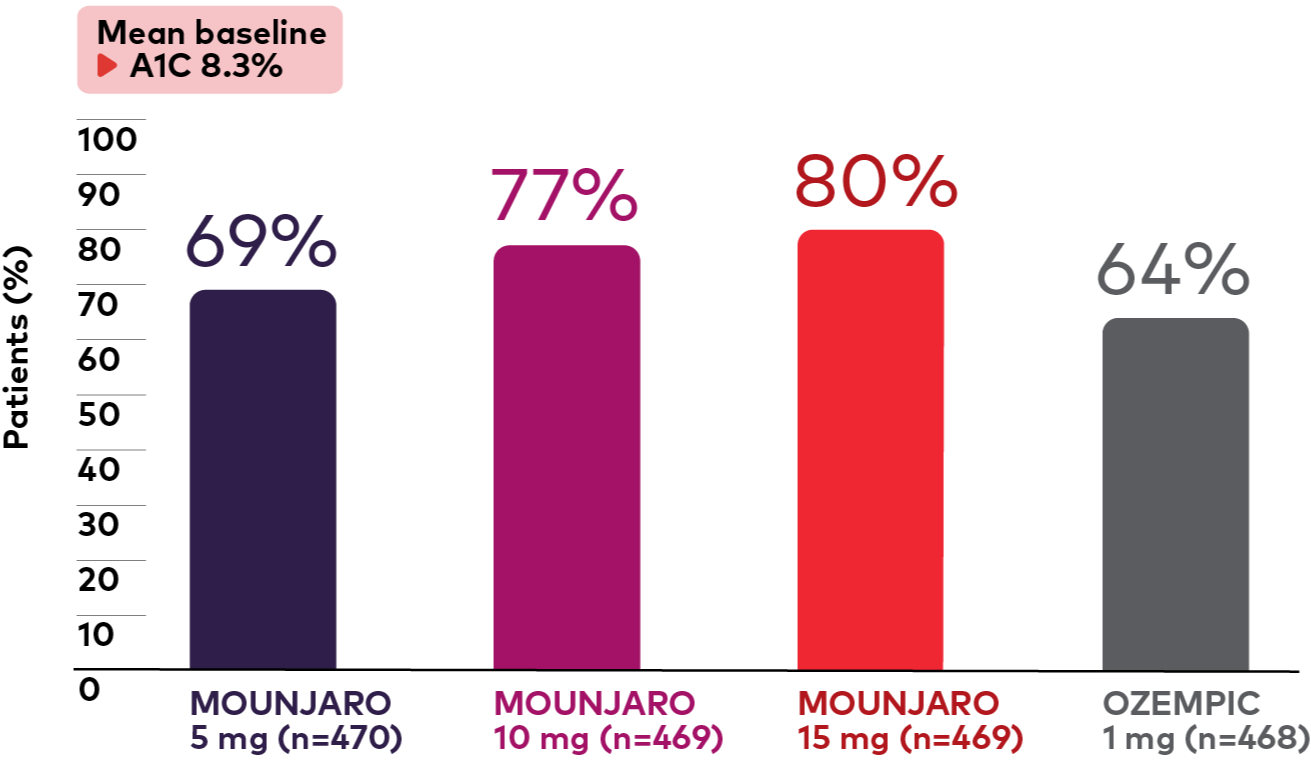
Bar graph showing the percentage of adult patients with type 2 diabetes on metformin in the SURPASS-2 study who had an A1C of less than or equal to 6.5% at 40 weeks. Graph shows Mounjaro vs Ozempic 1 mg. The bar graph shows the percentage of adult patients with type 2 diabetes on metformin in the SURPASS-2 study who had an A1C of less than 6.5% at 40 weeks. From a mean baseline A1C of 8.3%, 69% (Mounjaro 5 mg, n=470), 77% (Mounjaro 10 mg, n=469), 80% (Mounjaro 15 mg, n=469), and 64% (Ozempic 1 mg, n=468) of patients had an A1C of less than or equal to 6.5%.
Percentage of Patients With A1C <5.7% at 40 Weeks1,¶,#
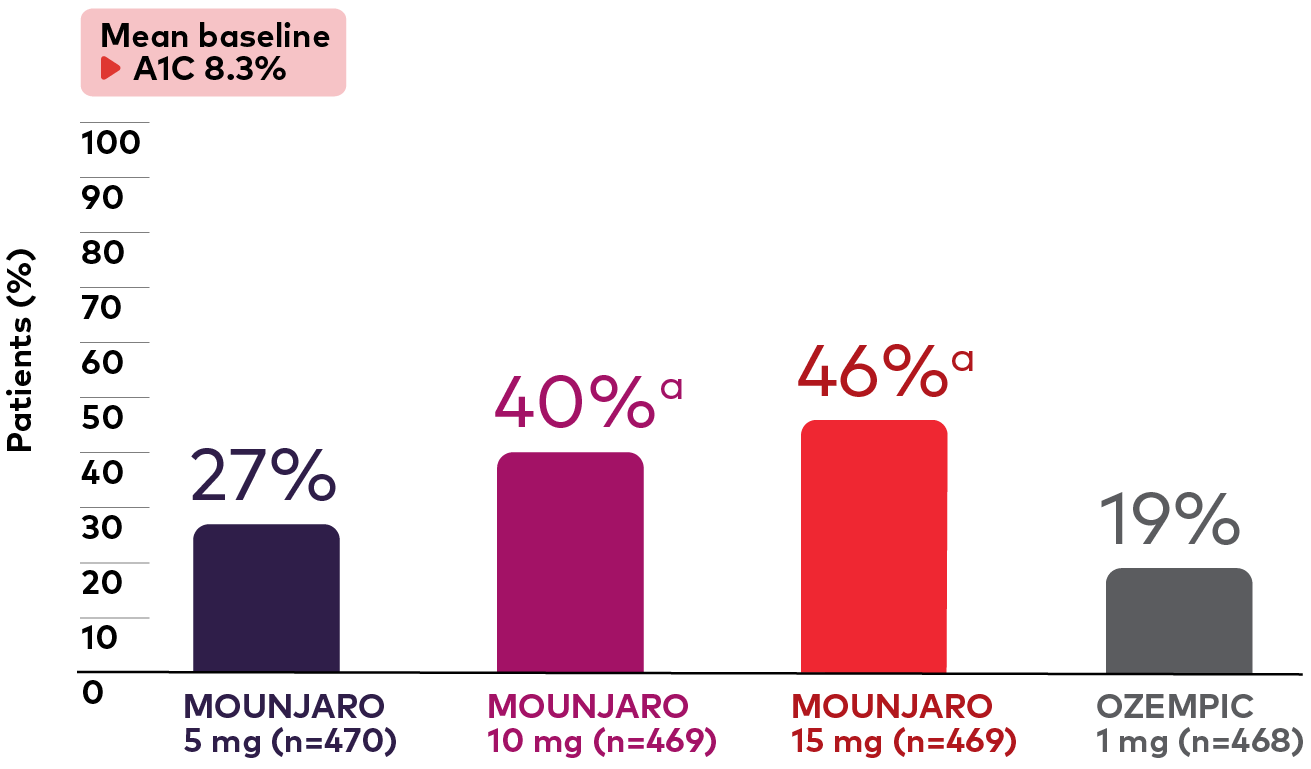
Bar graph showing the percentage of adult patients with type 2 diabetes on metformin in the SURPASS-2 study who had an A1C less than 5.7% at 40 weeks. Graph shows Mounjaro vs Ozempic 1 mg. The bar graph shows the percentage of adult patients with type 2 diabetes on metformin in the SURPASS-2 study who had an A1C of less than 5.7% at 40 weeks. From a mean baseline A1C of 8.3%, 27% (Mounjaro 5 mg, n=470), 40% (Mounjaro 10 mg, n=469), 46% (Mounjaro 15 mg, n=469), and 19% (Ozempic 1 mg, n=468) of patients had an A1C of less than 5.7%.
§Proportion of participants with A1C ≤6.5% was evaluated as an additional secondary endpoint not controlled for type I error. Logistic regression adjusted for baseline value and other stratification factors, with multiple imputation using retrieved dropout for missing value at 40 weeks.
¶Proportion of participants with A1C <5.7% was evaluated as a key secondary endpoint (controlled for type I error) for the 10-mg and 15-mg doses, and as an additional secondary endpoint (not controlled for type I error) for the 5-mg dose. Logistic regression adjusted for baseline value and other stratification factors, with multiple imputation using retrieved dropout for missing value at 40 weeks.
#A1C <5.7% is not a treatment goal supported by current treatment guidelines.
T2D=type 2 diabetes.
Select Important Safety Information
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Common adverse reactions in placebo-controlled trials1
Adverse reactions in pool of placebo-controlled trials reported in ≥5% of Mounjaro-treated patients1
| MOUNJARO 5 mg (n=237) | MOUNJARO 10 mg (n=240) | MOUNJARO 15 mg (n=241) | PLACEBO (n=235) | |
|---|---|---|---|---|
| Nausea | MOUNJARO 5 mg (n=237): 12% | MOUNJARO 10 mg (n=240): 15% | MOUNJARO 15 mg (n=241): 18% | PLACEBO (n=235): 4% |
| Diarrhea | MOUNJARO 5 mg (n=237): 12% | MOUNJARO 10 mg (n=240): 13% | MOUNJARO 15 mg (n=241): 17% | PLACEBO (n=235): 9% |
| Decreased appetite | MOUNJARO 5 mg (n=237): 5% | MOUNJARO 10 mg (n=240): 10% | MOUNJARO 15 mg (n=241): 11% | PLACEBO (n=235): 1% |
| Vomiting | MOUNJARO 5 mg (n=237): 5% | MOUNJARO 10 mg (n=240): 5% | MOUNJARO 15 mg (n=241): 9% | PLACEBO (n=235): 2% |
| Constipation | MOUNJARO 5 mg (n=237): 6% | MOUNJARO 10 mg (n=240): 6% | MOUNJARO 15 mg (n=241): 7% | PLACEBO (n=235): 1% |
| Dyspepsia | MOUNJARO 5 mg (n=237): 8% | MOUNJARO 10 mg (n=240): 8% | MOUNJARO 15 mg (n=241): 5% | PLACEBO (n=235): 3% |
| Abdominal pain | MOUNJARO 5 mg (n=237): 6% | MOUNJARO 10 mg (n=240): 5% | MOUNJARO 15 mg (n=241): 5% | PLACEBO (n=235): 4% |
The majority of reported nausea, vomiting, and/or diarrhea occurred during dose escalation and decreased over time1
This table shows common adverse reactions, excluding hypoglycemia, associated with the use of Mounjaro in the pool of phase 3 placebo-controlled trials. These adverse reactions occurred more commonly with Mounjaro than placebo and in at least 5% of patients treated with Mounjaro. Percentages reflect the number of patients who reported at least 1 treatment-emergent occurrence of the adverse reaction.
In the pool of placebo-controlled trials, gastrointestinal adverse reactions occurred more frequently among patients receiving Mounjaro than placebo (placebo 20.4%, Mounjaro 5 mg 37.1%, Mounjaro 10 mg 39.6%, Mounjaro 15 mg 43.6%).1
Percentage of patients who discontinued treatment due to GI adverse reactions, placebo-controlled trials1
| MOUNJARO 5 mg (n=237) | MOUNJARO 10 mg (n=240) | MOUNJARO 15 mg (n=241) | PLACEBO (n=235) | |
|---|---|---|---|---|
| Discontinuation rates | MOUNJARO 5 mg (n=237): 3.0% | MOUNJARO 10 mg (n=240): 5.4% | MOUNJARO 15 mg (n=241): 6.6% | PLACEBO (n=235): 0.4% |
Select Important Safety Information
Severe Gastrointestinal Disease: Use of Mounjaro has been associated with gastrointestinal adverse reactions, sometimes severe. Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
Incidence of hypoglycemia with Mounjaro in placebo-controlled trials1
Hypoglycemia adverse reactions in a placebo-controlled monotherapy trial, 40 weeks1||
| MOUNJARO 5 mg (n=121) | MOUNJARO 10 mg (n=119) | MOUNJARO 15 mg (n=120) | PLACEBO (n=115) | |
|---|---|---|---|---|
| Severe hypoglycemia** | MOUNJARO 5 mg (n=121): 0% | MOUNJARO 10 mg (n=119): 0% | MOUNJARO 15 mg (n=120): 0% | PLACEBO (n=115): 0% |
| Blood glucose level <54 mg/dL | MOUNJARO 5 mg (n=121): 0% | MOUNJARO 10 mg (n=191): 0% | MOUNJARO 15 mg (n=120): 0% | PLACEBO (n=115): 1% |
Hypoglycemia adverse reactions in a placebo-controlled trial as add-on to basal insulin with or without metformin, 40 weeks1||
| MOUNJARO 5 mg (n=116) | MOUNJARO 10 mg (n=119) | MOUNJARO 15 mg (n=120) | PLACEBO (n=120) | |
|---|---|---|---|---|
| Severe hypoglycemia** | MOUNJARO 5 mg (n=116): 0% | MOUNJARO 10 mg (n=119): 2% | MOUNJARO 15 mg (n=120): 1% | PLACEBO (n=120): 0% |
| Blood glucose level <54 mg/dL | MOUNJARO 5 mg (n=116): 16% | MOUNJARO 10 mg (n=119): 19% | MOUNJARO 15 mg (n=120): 14% | PLACEBO (n=120): 13% |
||Reflects the study treatment period. Data include events occurring during 4 weeks of treatment-free safety follow-up. Events after introduction of a new glucose-lowering treatment are excluded.
**Episodes requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.
Hypoglycemia was more frequent when Mounjaro was used in combination with a sulfonylurea. In a clinical trial up to 104 weeks of treatment, when administered with a sulfonylurea, severe hypoglycemia occurred in 0.5%, 0%, and 0.6%, and hypoglycemia (glucose level <54 mg/dL) occurred in 13.8%, 9.9%, and 12.8% of patients treated with Mounjaro 5 mg, 10 mg, and 15 mg, respectively.1
GI=gastrointestinal.
Select Important Safety Information
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Hypersensitivity Reactions: Serious hypersensitivity reactions (e.g., anaphylaxis and angioedema) have been reported in patients treated with Mounjaro. If hypersensitivity reactions occur, discontinue use of Mounjaro; treat promptly per standard of care, and monitor until signs and symptoms resolve. Do not use in patients with a previous serious hypersensitivity to tirzepatide or any of the excipients in Mounjaro. Use caution in patients with a history of angioedema or anaphylaxis with a GLP-1 receptor agonist because it is unknown if such patients will be predisposed to these reactions with Mounjaro.
Common adverse events in a trial of Mounjaro vs Ozempic 1 mg3
Adverse events occurring in ≥5% of Mounjaro-treated patients3
| MOUNJARO 5 mg (n=470) | MOUNJARO 10 mg (n=469) | MOUNJARO 15 mg (n=470) | OZEMPIC 1 mg (n=469) | |
|---|---|---|---|---|
| Nausea | MOUNJARO 5 mg (n=470): 17% | MOUNJARO 10 mg (n=469): 19% | MOUNJARO 15 mg (n=470): 22% | OZEMPIC 1 mg (n=469): 18% |
| Diarrhea | MOUNJARO 5 mg (n=470): 13% | MOUNJARO 10 mg (n=469): 16% | MOUNJARO 15 mg (n=470): 14% | OZEMPIC 1 mg (n=469): 12% |
| Vomiting | MOUNJARO 5 mg (n=470): 6% | MOUNJARO 10 mg (n=469): 9% | MOUNJARO 15 mg (n=470): 10% | OZEMPIC 1 mg (n=469): 8% |
| Dyspepsia | MOUNJARO 5 mg (n=470): 7% | MOUNJARO 10 mg (n=469): 6% | MOUNJARO 15 mg (n=470): 9% | OZEMPIC 1 mg (n=469): 7% |
| Decreased appetite | MOUNJARO 5 mg (n=470): 7% | MOUNJARO 10 mg (n=469): 7% | MOUNJARO 15 mg (n=470): 9% | OZEMPIC 1 mg (n=469): 5% |
| Abdominal pain | MOUNJARO 5 mg (n=470): 3% | MOUNJARO 10 mg (n=469): 5% | MOUNJARO 15 mg (n=470): 5% | OZEMPIC 1 mg (n=469): 5% |
| Constipation | MOUNJARO 5 mg (n=470): 7% | MOUNJARO 10 mg (n=469): 5% | MOUNJARO 15 mg (n=470): 5% | OZEMPIC 1 mg (n=469): 6% |
Percentage of patients who discontinued treatment due to GI Adverse Events4
| MOUNJARO 5 mg (n=470) | MOUNJARO 10 mg (n=469) | MOUNJARO 15 mg (n=470) | OZEMPIC 1 mg (n=469) | |
|---|---|---|---|---|
| Discontinuation rate | MOUNJARO 5 mg (n=470): 2.8% | MOUNJARO 10 mg (n=469): 4.3% | MOUNJARO 15 mg (n=470): 4.3% | OZEMPIC 1 mg (n=469): 3.2% |
Hypoglycemic Adverse Events in a 40-Week Trial of Mounjaro vs. Ozempic 1 mg3
| Add-on to metformin | Mounjaro 5 mg | Mounjaro 10 mg | Mounjaro 15 mg | Ozempic 1 mg |
|---|---|---|---|---|
| Severe Hypoglycemia†† | Mounjaro 5 mg (n=470): 0.2% | Mounjaro 10 mg (n=469): 0% | Mounjaro 15 mg (n=470): 0.2%‡‡ | Ozempic 1 mg (n=469): 0% |
| Blood glucose level <54 mg/dL | Mounjaro 5 mg (n=470): 0.6% | Mounjaro 10 mg (n=469): 0.2% | Mounjaro 15 mg (n=470): 1.7% | Ozempic 1 mg (n=469): 0.4% |
SURPASS-2 was not designed to evaluate the relative safety between Mounjaro and Ozempic 1 mg. Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.1
††Episodes requiring the assistance of another person to actively administer carbohydrate, glucose, or other resuscitative actions.
‡‡One patient had a hypoglycemic event that was not considered by the investigator to be severe, but it was reported as a serious adverse event.
GI=gastrointestinal.
Select Important Safety Information
Acute Kidney Injury: Mounjaro has been associated with gastrointestinal adverse reactions, which include nausea, vomiting, and diarrhea. These events may lead to dehydration, which if severe could cause acute kidney injury. In patients treated with GLP-1 receptor agonists, there have been postmarketing reports of acute kidney injury and worsening of chronic renal failure, sometimes requiring hemodialysis. Some of these events have been reported in patients without known underlying renal disease. A majority of reported events occurred in patients who had experienced nausea, vomiting, diarrhea, or dehydration. Monitor renal function when initiating or escalating doses of Mounjaro in patients with renal impairment reporting severe adverse gastrointestinal reactions.
Severe Gastrointestinal Disease: Use of Mounjaro has been associated with gastrointestinal adverse reactions, sometimes severe. Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
Mounjaro vs Ozempic (+ metformin) Study Design
- SURPASS-2 was a 40-week, open-label (double-blind with respect to Mounjaro dose assignment), active-controlled, phase 3 trial that randomized 1879 adult patients with T2D who had inadequate glycemic control on stable doses of metformin alone to receive once-weekly SC Mounjaro 5 mg, 10 mg, or 15 mg or once-weekly SC Ozempic® 1 mg (1:1:1:1 ratio), all in combination with metformin ≥1500 mg per day1,3
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg in Ozempic in mean change from baseline in A1C at 40 weeks3
- The key secondary objectives were assessed at 40 weeks: noninferiority of Mounjaro 5 mg to Ozempic in mean change from baseline in A1C; superiority of Mounjaro to Ozempic in mean change from baseline in A1C; superiority of proportion of patients with A1C <7%; superiority in mean change from baseline in weight; superiority of Mounjaro 10 mg and/or 15 mg to Ozempic in proportion of patients with A1C <5.7%3
- Study participants had a mean baseline A1C of 8.3% and a mean T2D duration of 8.6 years1,3
SC=subcutaneous.

Discover Lilly Play
Watch videos about Mounjaro and how to help your patients get started.
Unmatched weight reduction across all 3 doses vs Ozempic 1 mg§§
Mounjaro is not indicated for weight loss.
Change in weight was a secondary endpoint.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Change in weight from baseline across doses (lb)1,9
Mounjaro vs Ozempic 1 mg
Study length: 40 weeks
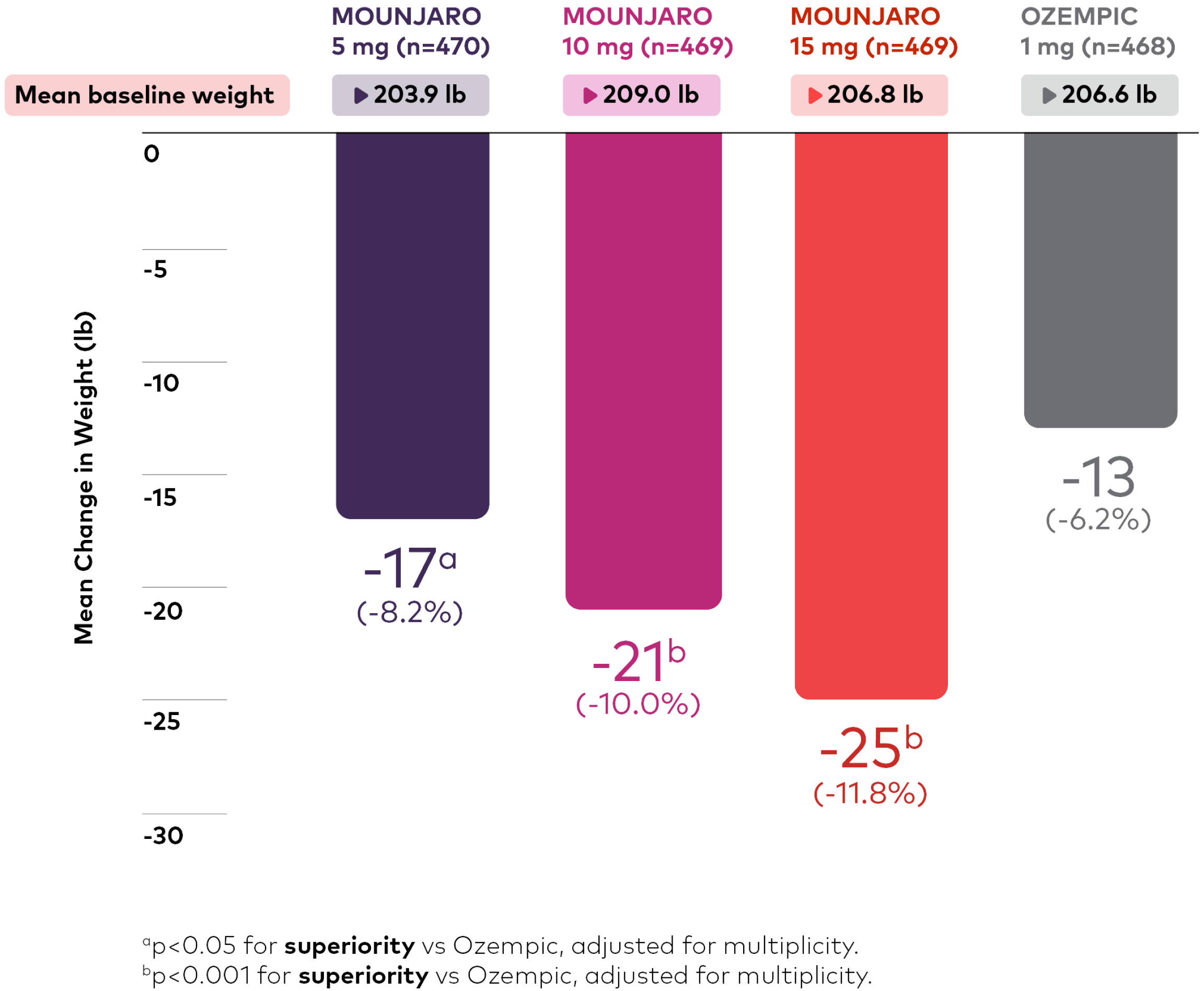
Bar chart depicting change in weight from baseline at 40 weeks. There were 470, 469, 469, and 468 patients in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and the mean baseline weights were 203.9 lb, 209.0 lb, 206.8 lb, and 206.6 lb, respectively. Mean changes in weight at 40 weeks were -17 lb or -8.2% (p<0.05 for superiority vs. Ozempic, adjusted for multiplicity), -21 lb or -10.0% (p<0.001 for superiority vs. Ozempic, adjusted for multiplicity), -25 lb or -11.8% (p<0.001 for superiority vs. Ozempic, adjusted for multiplicity), and -13 lb or -6.2% for Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg, respectively.
§§In other studies of glycemic control with a primary endpoint at 40 weeks or 52 weeks, mean reductions in body weight ranged from 12 lb to 15 lb for the 5-mg dose, 15 lb to 21 lb for the 10-mg dose, and 17 lb to 25 lb for the 15-mg dose; and for comparators, mean change was -2 lb and +4 lb for placebo, +4 lb for Tresiba®, and +4 lb for insulin glargine.1
Select Important Safety Information
Severe Gastrointestinal Disease: Use of Mounjaro has been associated with gastrointestinal adverse reactions, sometimes severe. Mounjaro has not been studied in patients with severe gastrointestinal disease, including severe gastroparesis, and is therefore not recommended in these patients.
Drug Interactions: When initiating Mounjaro, consider reducing the dose of concomitantly administered insulin secretagogues (such as sulfonylureas) or insulin to reduce the risk of hypoglycemia. Mounjaro delays gastric emptying, and thereby has the potential to impact the absorption of concomitantly administered oral medications, so caution should be exercised.
Patients taking Mounjaro experienced sustained weight reductions through 40 weeks1, 3, 10, ¶¶, ##
Mounjaro is not indicated for weight loss.
Change in weight was a secondary endpoint.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Observed mean change in weight over time from baseline to 40 weeks (lb)1,3,10
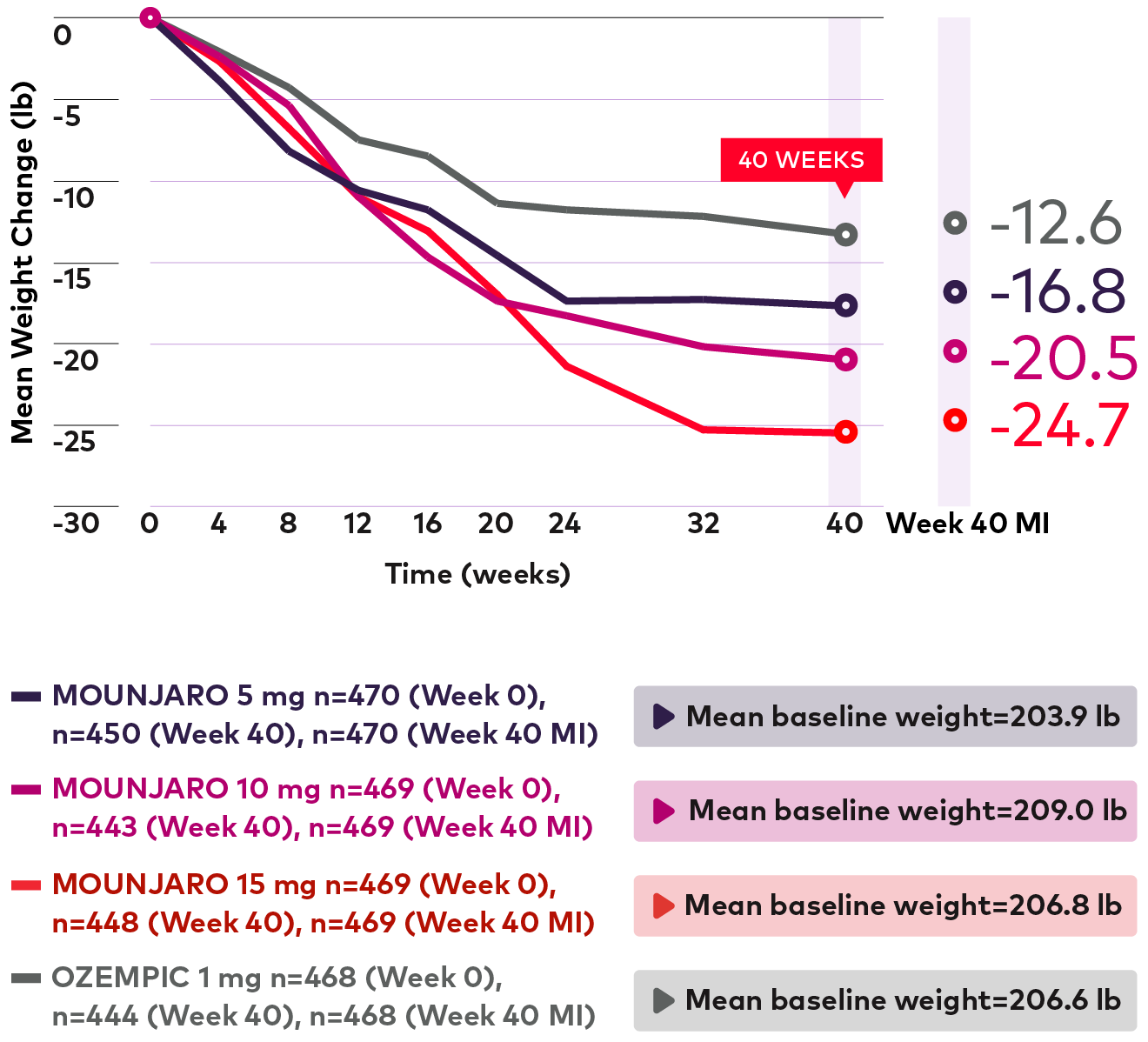
Line graph showing observed mean weight changes over time from 0 to 40 weeks followed by an additional calculation of multiple imputation at 40 weeks. For Mounjaro 5 mg, 470 patients were randomized with a mean baseline weight of 204.0 pounds. 450 patients completed the study. Weight at week 40 for MI value of 470 patients was -16.8 pounds. For Mounjaro 10 mg, 469 patients were randomized with a mean baseline weight of 209.0 pounds. 443 patients completed the study. Weight at week 40 for MI value of 469 patients was -20.5 pounds. For Mounjaro 15 mg, 469 patients were randomized with a mean baseline weight of 206.8 pounds. 448 patients completed the study. Weight at week 40 for MI value of 468 patients was -24.7 pounds. For Ozempic 1 mg, 468 patients were randomized with a mean baseline weight of 206.6 pounds. 444 patients completed the study. Weight at week 40 for MI value of 468 patients was -12.6 pounds.
Patients taking Mounjaro had weight reductions¶¶, ## that continued through 40 weeks1,3,10
¶¶In other studies of glycemic control with a primary endpoint at 40 weeks or 52 weeks, mean reductions in body weight ranged from 12 lb to 15 lb for the 5-mg dose; 15 lb to 21 lb for the 10-mg dose, and 17 lb to 25 lb for the 15-mg dose; and for comparators, mean change was -2 lb and +4 lb for placebo, +4 lb for Tresiba®, and +4 lb for insulin glargine.1
##Data represent observed mean changes from week 0 to week 40, and least-squares mean at week 40 MI. mITT population, full analysis set. ANCOVA was performed for change from baseline at week 40 and MMRM analysis was performed for change over time.
Select Important Safety Information
Pregnancy: Limited data on Mounjaro use in pregnant women are available to inform on drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Based on animal reproduction studies, there may be risks to the fetus from exposure to tirzepatide. Use only if potential benefit justifies the potential risk to the fetus.

Want to learn more about Mounjaro's clinical data from a peer?
See if you qualify for a virtual program by entering your NPI below
By providing my professional information, I agree that it may be used by Eli Lilly and Company (“Lilly”) and its affiliates and partners or third parties, working on Lilly's behalf, for Lilly's marketing or promotional purposes. I may unsubscribe/opt out by clicking the unsubscribe link within any email I receive or by calling 1-800-LILLYRX (1-800-545-5979). Available Mon-Fri, 9 am-8 pm ET. For more information about Lilly's privacy practices, please view the Privacy Statement.
Percentage of patients with weight reduction at 40 weeks3
Mounjaro is not indicated for weight loss.
IN ADULT PATIENTS WITH T2D ON METFORMIN
Percentage of patients with weight reduction ≥10%1,3,***
Mounjaro vs Ozempic 1 mg
Study length: 40 weeks
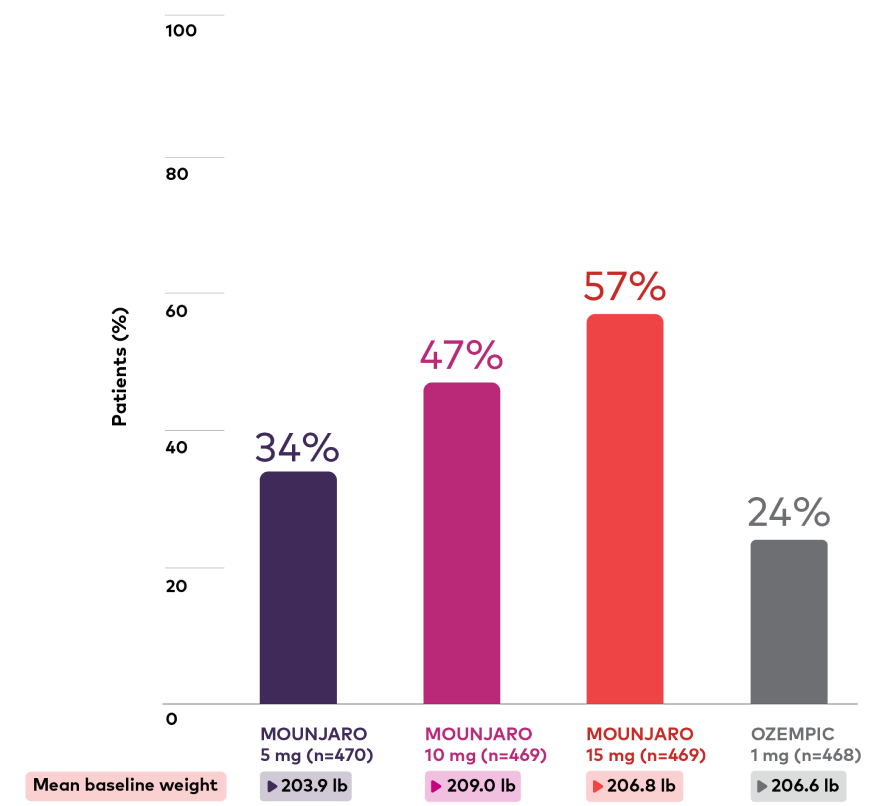
Bar chart depicting proportion of patients with weight reduction ≥10% at 40 weeks. There were 470, 469, 469, and 468 patients in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and the mean baseline weights were 203.9 lb, 209.0 lb, 206.8 lb, and 206.6 lb, respectively. The proportion with weight reduction ≥10% at 40 weeks was 34%, 47%, 57%, and 24% for Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg, respectively.
Percentage of patients with weight reduction ≥15%1,3,***
Mounjaro vs Ozempic 1 mg
Study length: 40 weeks
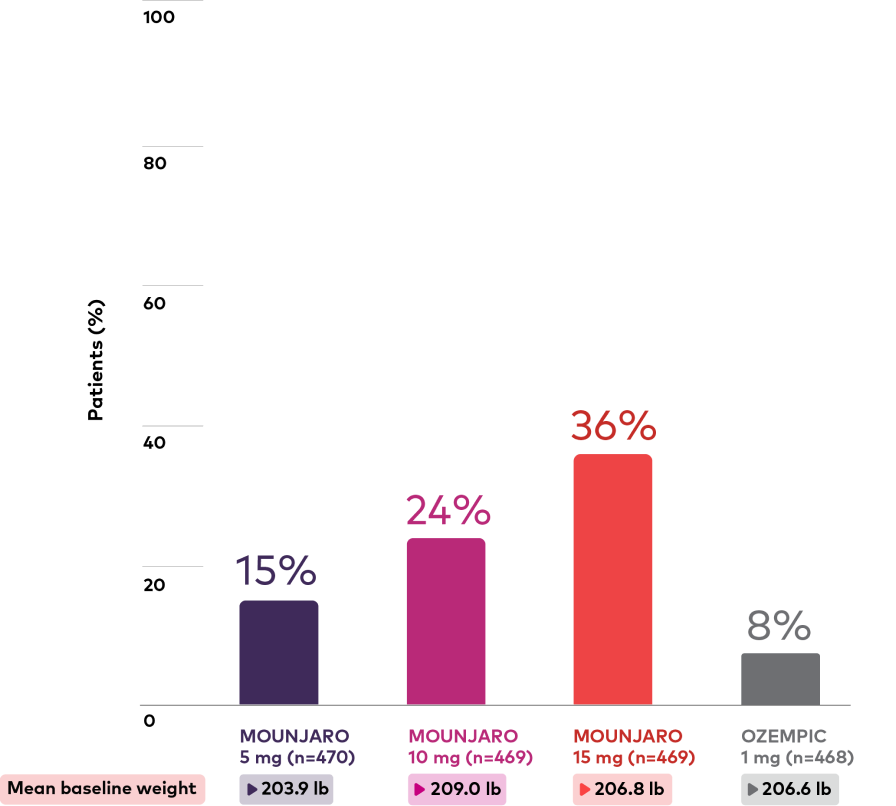
Bar chart depicting proportion of patients with weight reduction ≥15% at 40 weeks. There were 470, 469, 469, and 468 patients in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and the mean baseline weights were 203.9 lb, 209.0 lb, 206.8 lb, and 206.6 lb, respectively. The proportion with weight reduction ≥15% at 40 weeks was 15%, 24%, 36%, and 8% for Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg, respectively.
***In clinical studies, the percentage of patients with weight reduction ≥10% and ≥15% were additional secondary endpoints not controlled for type I error. Proportions were determined using logistic regression with multiple imputation using retrieved dropout for missing value at 40 weeks.
Lactation: There are no data on the presence of tirzepatide in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Mounjaro and any potential adverse effects on the breastfed infant from Mounjaro or from the underlying maternal condition.
Mounjaro vs Ozempic (+ metformin) Study Design
- SURPASS-2 was a 40-week, open-label (double-blind with respect to Mounjaro dose assignment), active-controlled, phase 3 trial that randomized 1879 adult patients with T2D who had inadequate glycemic control on stable doses of metformin alone to receive once-weekly SC Mounjaro 5 mg, 10 mg, or 15 mg or once-weekly SC Ozempic 1 mg (1:1:1:1 ratio), all in combination with metformin ≥1500 mg per day1,3
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg in Ozempic in mean change from baseline in A1C at 40 weeks3
- The key secondary objectives were assessed at 40 weeks: noninferiority of Mounjaro 5 mg to Ozempic in mean change from baseline in A1C; superiority of Mounjaro to Ozempic in mean change from baseline in A1C; superiority of proportion of patients with A1C <7%; superiority in mean change from baseline in weight; superiority of Mounjaro 10 mg and/or 15 mg to Ozempic in proportion of patients with A1C <5.7%33
- Study participants had a mean baseline A1C of 8.3% and a mean T2D duration of 8.6 years1,3
Subgroup analysis of mean weight change by baseline BMI11
Mounjaro is not indicated for weight loss.
Post Hoc Analysis: Mean weight change from baseline (40 weeks)11
Patients with Baseline BMI <30 kg/m2
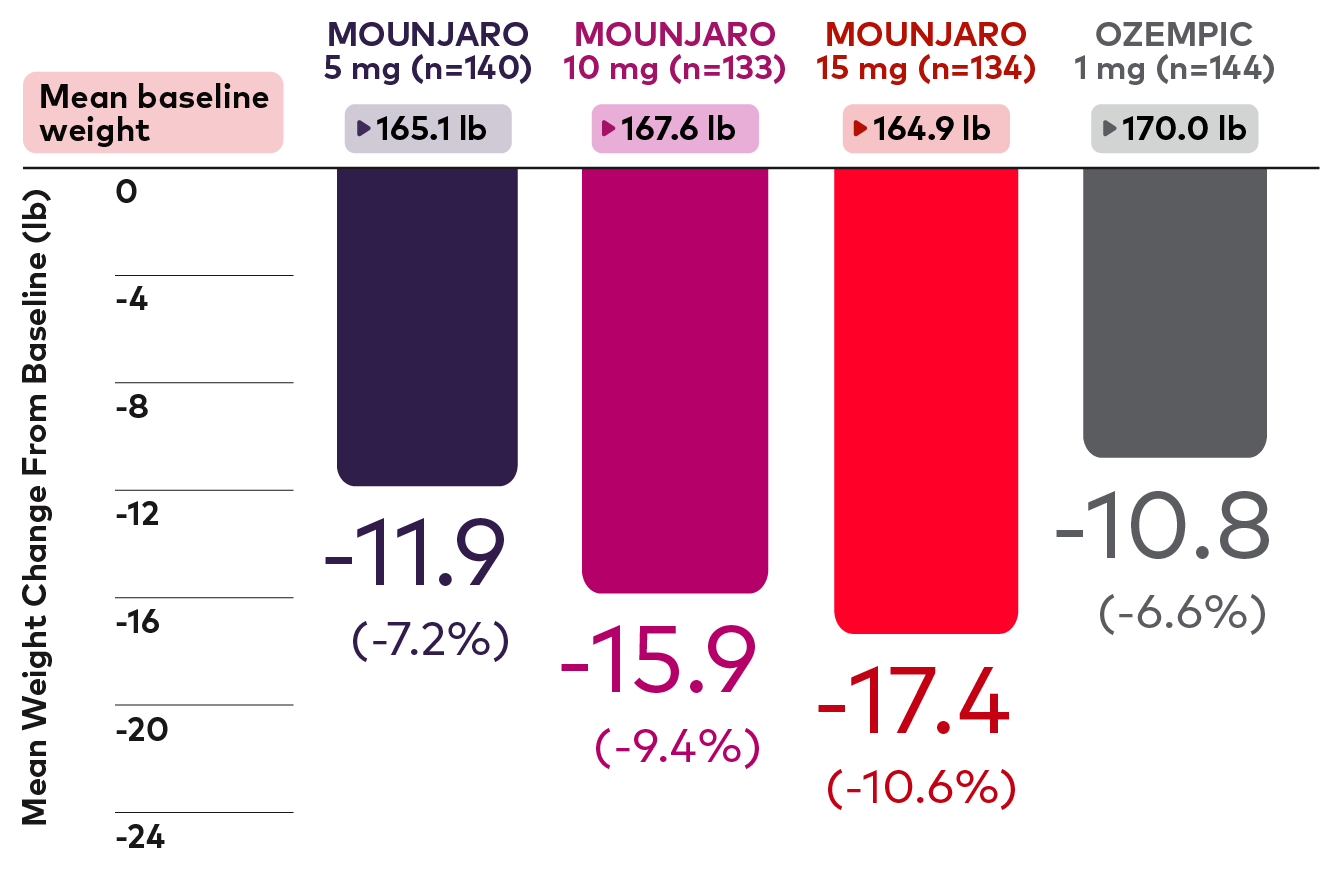
Bar graph showing the absolute and percentage mean weight change in patients with a baseline BMI of <30 kg/m2 at 40 weeks in the SURPASS-2 trial (Mounjaro 5 mg, 10 mg, and 15 mg vs Ozempic 1 mg). Mean changes were -11.9 lb (-7.2%) for Mounjaro 5 mg (n=140), -15.9 lb (-9.4%) for Mounjaro 10 mg (n=133), -17.4 lb (-10.6%) for Mounjaro 15 mg (n=134), and -10.8 lb (-6.6%) for Ozempic 1 mg (n=144) from mean baseline weights of 165.1 lb, 167.6 lb, 164.9 lb, and 170.0 lb, respectively.
Patients with baseline BMI ≥30 kg/m2:
The mean weight change of patients with a baseline BMI of ≥30 kg/m2 was -18.7 lb (-8.6%) for Mounjaro 5 mg (n=330), -22.5 lb (-10.3%) for Mounjaro 10 mg (n=336), and -27.6 lb (-12.3%) for Mounjaro 15 mg (n=335) vs -13.2 lb (-6.1%) for Ozempic 1 mg (n=324) from mean baseline weights of 220.2 lb, 225.5 lb, 223.5 lb, and 222.9 lb, respectively.
Mounjaro is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
Limitations of Use: Mounjaro has not been studied in patients with a history of pancreatitis. Mounjaro is not indicated for use in patients with type 1 diabetes mellitus.
In SURPASS-2, change in weight by baseline BMI at 40 weeks was a secondary endpoint not controlled for type I error.
LSM change was estimated using MMRM with treatment, visit, treatment-by-visit interaction, pooled country, baseline A1C group, baseline OAM (when appropriate), and baseline weight as fixed effects, and patient as random effect.12
BMI=body mass index; LSM=least-squares mean; MMRM=mixed model for repeated measures; OAM=oral antihyperglycemic medication.
Help get your patients started on Mounjaro
Composite endpoint of A1C ≤6.5%, weight reduction ≥10%, and no clinically significant or severe hypoglycemia3,13,†††
Mounjaro is not indicated for weight loss.
Composite Endpoint Results With Mounjaro vs Ozempic 1 mg
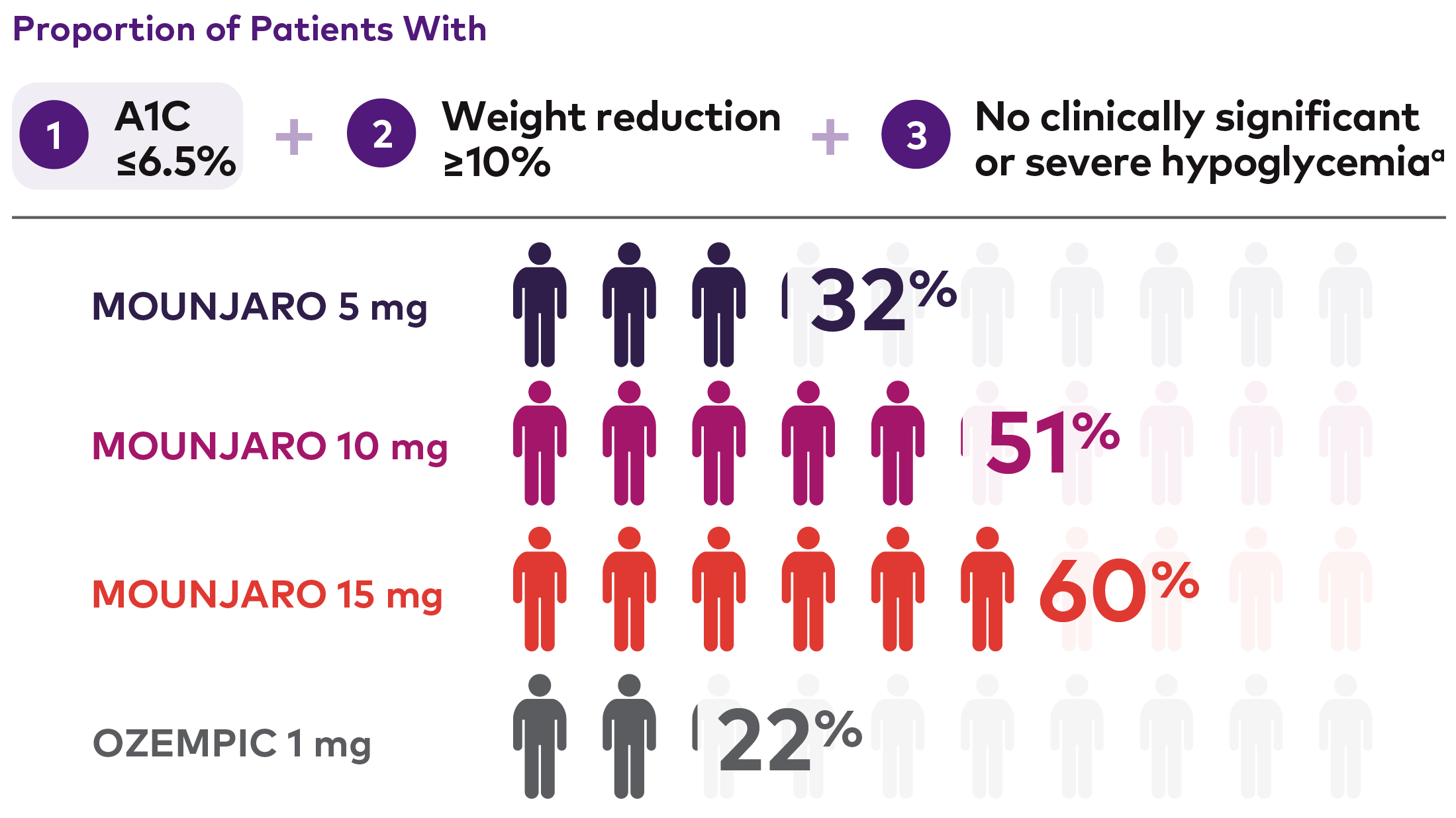
Figure depicting proportion of patients with a composite endpoint of A1C ≤6.5% + weight reduction ≥10% + no clinically significant or severe hypoglycemic events at 40 weeks. There were 470, 469, 469, and 468 patients in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and 32%, 51%, 60%, and 22% of patients, respectively, had this composite endpoint at 40 weeks.
aClinically significant hypoglycemia defined as plasma glucose <54 mg/dL. Severe hypoglycemia defined as episodes requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions.3
†††In SURPASS-2, composite outcome was a prespecified secondary endpoint not controlled for type I error. Analysis based on efficacy estimand data (on-treatment efficacy without the influence of rescue therapy) and may not represent a real-world setting. The number of patients included in the efficacy analysis data set for Mounjaro 5 mg, 10 mg, 15 mg and Ozempic 1 mg were 470, 469, 469, and 468, respectively. Only participants with baseline value and at least one post-baseline value for the response variables were included in the analysis.13
Select Important Safety Information
Hypoglycemia with Concomitant Use of Insulin Secretagogues or Insulin: Concomitant use with an insulin secretagogue (e.g., sulfonylurea) or insulin may increase the risk of hypoglycemia, including severe hypoglycemia. The risk of hypoglycemia may be lowered by reducing the dose of sulfonylurea (or other concomitantly administered insulin secretagogue) or insulin. Inform patients using these concomitant medications of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
Mounjaro vs Ozempic (+ metformin) Study Design
- SURPASS-2 was a 40-week, open-label (double-blind with respect to Mounjaro dose assignment), active-controlled, phase 3 trial that randomized 1879 adult patients with T2D who had inadequate glycemic control on stable doses of metformin alone to receive once-weekly SC Mounjaro 5 mg, 10 mg, or 15 mg or once-weekly SC Ozempic 1 mg (1:1:1:1 ratio), all in combination with metformin ≥1500 mg per day11,3
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg to Ozempic in mean change from baseline in A1C at 40 weeks3
- The key secondary objectives were assessed at 40 weeks: noninferiority of Mounjaro 5 mg to Ozempic in mean change from baseline in A1C; superiority of Mounjaro to Ozempic in mean change from baseline in A1C; superiority of proportion of patients with A1C <7%; superiority in mean change from baseline in weight; superiority of Mounjaro 10 mg and/or 15 mg to Ozempic in proportion of patients with A1C <5.7%3
- Study participants had a mean baseline A1C of 8.3% and a mean T2D duration of 8.6 years1,3
Mounjaro delivered superior A1C reductions when studied across a range of background therapies and comparators1,‡‡‡
A1C reductions were consistently seen across studies vs Ozempic 1 mg1, Tresiba® (insulin degludec)a, insulin glargineb, and placebo.
Reductions in A1C From Baseline (%) Across Doses and Trials1
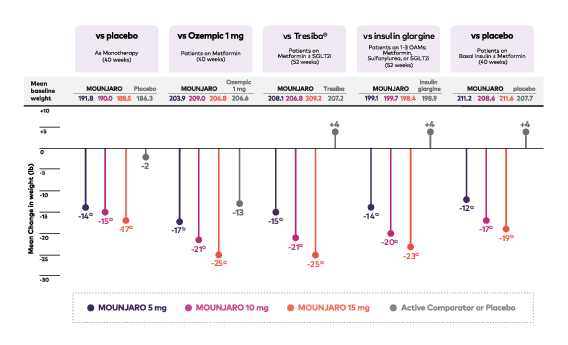
Panel of 5 lollipop graphs showing the mean change in A1C at primary endpoint for adults with type 2 diabetes in the SURPASS 1-5 studies. The first graph shows Mounjaro vs placebo as monotherapy. From a mean baseline A1C of 8.0% (Mounjaro 5 mg, n=121), 7.9% (Mounjaro 10 mg, n=121), 7.9% (Mounjaro 15 mg, n=120), and 8.1% (placebo, n=113), mean change in A1C at 40 weeks was -1.8%, -1.7%, -1.7%, and -0.1%, respectively.
The second graph shows Mounjaro vs Ozempic 1 mg as add-on to metformin. From a mean baseline A1C of 8.3% in all treatment arms (Mounjaro 5 mg, n=470; Mounjaro 10 mg, n=469; Mounjaro 15 mg, n=469; and Ozempic 1 mg, n=468), mean change in A1C at 40 weeks was -2.0%, -2.2%, -2.3%, and -1.9%, respectively.
The third graph shows Mounjaro vs Tresiba as add-on to metformin with or without SGLT2i. From a mean baseline A1C of 8.2% in all Mounjaro treatment arms (Mounjaro 5 mg, n=358; Mounjaro 10 mg, n=360; Mounjaro 15 mg, n=358) and 8.1% for Tresiba (n=359), mean change in A1C at 52 weeks was -1.9%, -2.0%, -2.1%, and -1.3%, respectively.
The fourth graph shows Mounjaro vs insulin glargine as add-on to 1-3 oral antihyperglycemic medications (metformin, SGLT2i, or sulfonylurea). From a mean baseline A1C of 8.5% (Mounjaro 5 mg, n=328), 8.6% (Mounjaro 10 mg, n=326), 8.5% (Mounjaro 15 mg, n=337), and 8.5% (insulin glargine, n=998), mean change in A1C at 52 weeks was -2.1%, -2.3%, -2.4%, and -1.4%, respectively.
The fifth graph shows Mounjaro vs placebo as add-on to basal insulin with or without metformin. From a mean baseline A1C of 8.3% (Mounjaro 5 mg, n=116), 8.4% (Mounjaro 10 mg, n=118), 8.2% (Mounjaro 15 mg, n=118), and 8.4% (placebo, n=119), mean change in A1C at 40 weeks was -2.1%, -2.4%, -2.3%, and -0.9%, respectively.
Data represent least-squares mean from ANCOVA adjusted for baseline value and other stratification factors.
aAt week 52, 26% of patients randomized to Tresiba achieved the fasting serum glucose target of <90 mg/dL, and the mean daily Tresiba dose was 49 U (0.5 U/kg).1
bAt week 52, 30% of patients randomized to insulin glargine achieved the fasting serum glucose target of <100 mg/dL, and the mean daily insulin glargine dose was 44 U (0.5 U/kg).1
cp<0.001 for superiority vs comparator, adjusted for multiplicity.
dp<0.05 for superiority vs Ozempic 1 mg, adjusted for multiplicity.
‡‡‡SURPASS study participants were adults on monotherapy, up to 3 orals, or basal insulin. They had a mean baseline A1C that ranged from 7.9% to 8.6%, and a mean duration of T2D that ranged from 4.7 to 13.3 years.1
ANCOVA=analysis of covariance; OAM=oral antihyperglycemic medication; SGLT2i=sodium-glucose co-transporter 2 inhibitor; T2D=type 2 diabetes.
Mounjaro demonstrated significant weight results across 5 clinical trials1
Mounjaro consistently demonstrated weight reduction in adult patients with type 2 diabetes across studies vs Ozempic 1 mg, Tresiba, insulin glargine, and placebo.1
Mounjaro is not indicated for weight loss.
Change in weight was a secondary endpoint.
Change in Weight From Baseline Across Doses (lb)1
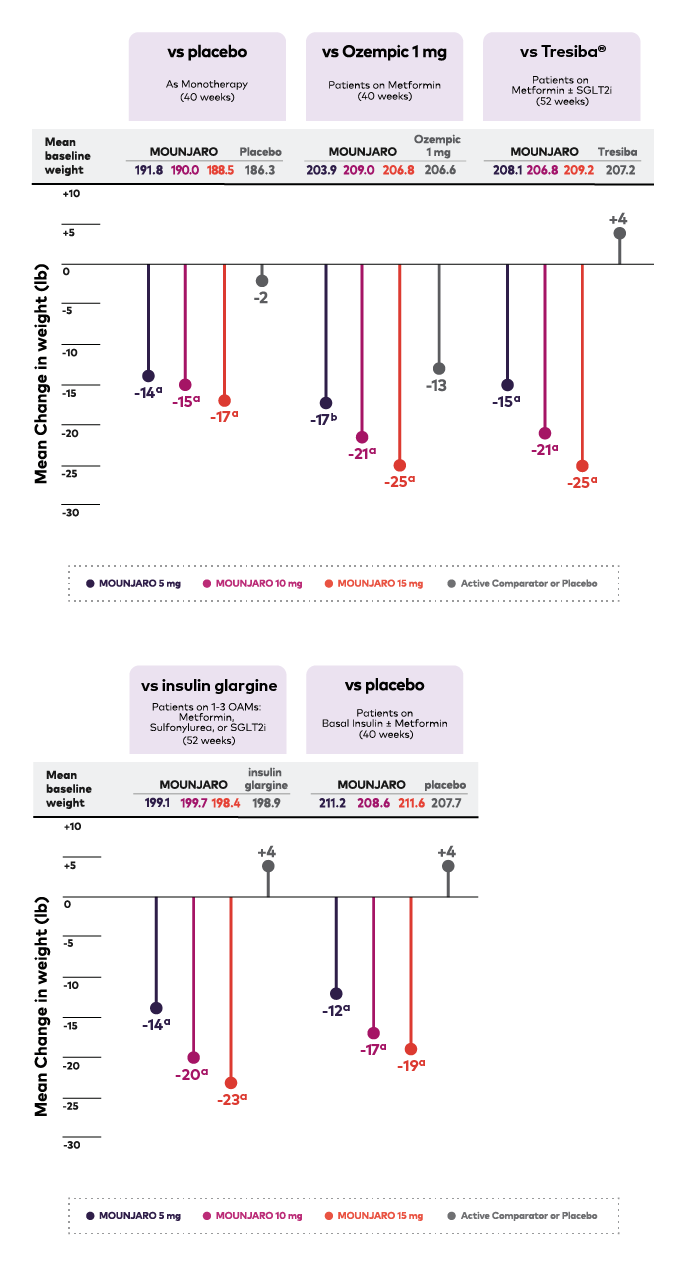
Chart depicting change in weight from baseline in pounds across doses and trials. In the trial of Mounjaro versus placebo as monotherapy, the mean change in weight from baseline in pounds was –14, -15, -17, and -2 in the Mounjaro 5 mg, 10 mg, 15 mg, and placebo treatment groups, respectively, and the mean baseline weight in pounds was 191.8, 190.0, 188.5, and 186.3 respectively. In the trial of Mounjaro versus Ozempic 1 mg with patients on Metformin, the mean baseline weight in pounds was -17, -21, -25, and -13 in the Mounjaro 5 mg, 10 mg, 15 mg, and Ozempic 1 mg treatment groups, respectively, and the mean baseline weight in pounds was 203.9, 209.0, 206.8, and 206.6 respectively. In the trial of Mounjaro versus Tresiba with patients on Metformin and or a SGLT2, the mean change in weight from baseline in pounds was -15, -21, -25, and +4 in the Mounjaro 5 mg, 10 mg, 15 mg, and Tresiba treatment group, respectively, and the mean baseline weight in pounds were 208.1, 206.8, 209.2, and 207.2 respectively. In the trial of Mounjaro versus Insulin Glargine with patients on 1-3 OAMs Metformin, Sulfonylurea, or SGLT2, the mean change in weight from baseline in pounds was -14, -20, -23, and +4 in the Mounjaro 5 mg, 10 mg, 15 mg, and Insulin Glargine treatment groups, respectively, and the mean baseline weight in pounds were 199.1, 199.7, 198.4, and 198.9 respectively. In the trial of Mounjaro versus placebo with patients on Basal Insulin and or Metformin, the mean change in weight from baseline in pounds was -12, -17, -19, and +4 in the Mounjaro 5 mg, 10 mg, 15 mg, and placebo treatment groups, respectively, and the mean baseline weight in pounds were 211.2, 208.6, 211.6, 207.7, respectively.
OAM=oral antihyperglycemic medication; SGLT2i=sodium-glucose co-transporter 2 inhibitor.
Select Important Safety Information
Pregnancy: Limited data on Mounjaro use in pregnant women are available to inform on drug-associated risk for major birth defects, miscarriage, or other adverse maternal or fetal outcomes. Based on animal reproduction studies, there may be risks to the fetus from exposure to tirzepatide. Use only if potential benefit justifies the potential risk to the fetus.
Study Designs
Mounjaro vs placebo
- SURPASS-1 was a 40-week, double-blind, placebo-controlled, phase 3 trial that randomized 478 adult patients with T2D who had inadequate glycemic control with diet and exercise to receive once-weekly SC Mounjaro 5 mg, 10 mg, or 15 mg, or placebo (1:1:1:1 ratio)1,5
- The primary objective was to demonstrate superiority of Mounjaro 5 mg, 10 mg, and/or 15 mg to placebo in mean change from baseline in A1C at 40 weeks5
- The key secondary objectives were assessed at 40 weeks: superiority of Mounjaro 5 mg, 10 mg, and/or 15 mg to placebo in proportion of patients with A1C <7% and <5.7%, mean change from baseline in fasting serum glucose, and mean change from baseline in weight5
- Study participants had a mean baseline A1C of 7.9% and mean T2D duration of 4.7 years1,5
Mounjaro vs Ozempic (+ metformin)
- SURPASS-2 was a 40-week, open-label (double-blind with respect to Mounjaro dose assignment), active-controlled, phase 3 trial that randomized 1879 adult patients with T2D who had inadequate glycemic control on stable doses of metformin alone to receive once-weekly SC Mounjaro 5 mg, 10 mg, or 15 mg or once-weekly SC Ozempic® 1 mg (1:1:1:1 ratio), all in combination with metformin ≥1500 mg per day1,3
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg to Ozempic in mean change from baseline in A1C at 40 weeks3
- The key secondary objectives were assessed at 40 weeks: noninferiority of Mounjaro 5 mg to Ozempic in mean change from baseline in A1C; superiority of Mounjaro to Ozempic in mean change from baseline in A1C; superiority of proportion of patients with A1C <7%; superiority in mean change from baseline in weight; superiority of Mounjaro 10 mg and/or 15 mg to Ozempic for proportion of patients with A1C <5.7%3
- Study participants had a mean baseline A1C of 8.3% and a mean T2D duration of 8.6 years1,3
Mounjaro vs Tresiba (+ metformin ± SGLT2i)
- SURPASS-3 was a 52-week, open-label, active-controlled, phase 3 trial that randomized 1444 adult patients with T2D who had inadequate glycemic control on stable doses of metformin with or without an SGLT2i to once-weekly SC Mounjaro 5 mg, 10 mg, 15 mg, or once-daily SC Tresiba 100 U/mL (1:1:1:1 ratio)1,6
- Tresiba was initiated at 10 U/day and titrated to a fasting blood glucose of <90 mg/dL using a treat-to-target algorithm. At week 52, the mean daily Tresiba dose was 49 U (0.5 U/kg), and 26% of patients achieved the target fasting serum glucose.6
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg to Tresiba in mean change from baseline in A1C at 52 weeks6
- The key secondary objectives were assessed at 52 weeks: noninferiority of Mounjaro 5 mg to Tresiba in mean change from baseline in A1C and superiority of Mounjaro 5 mg, 10 mg, and/or 15 mg to Tresiba in mean change from baseline in A1C, mean change from baseline in weight, and proportion of patients with A1C <7%6
- Study participants had a mean baseline A1C of 8.2% and a mean T2D duration of 8.4 years6
Mounjaro vs insulin glargine (+ 1-3 OAMs)
- SURPASS-4 was a 104-week, open-label, active-controlled, phase 3 trial that randomized 2002 adult patients with T2D who had increased cardiovascular risk to once-weekly SC Mounjaro 5 mg, 10 mg, 15 mg, or once-daily SC insulin glargine 100 U/mL (1:1:1:3 ratio), on background metformin (95%), and/or sulfonylureas (54%) and/or an SGLT2i (25%)1,7
- Insulin glargine was initiated at 10 U/day and titrated to a fasting blood glucose of <100 mg/dL using a treat-to-target algorithm; dose adjustments were made based on the median value of the last 3 self-monitored fasting blood glucose values. At week 52, 30% of patients achieved the target fasting serum glucose, and the mean daily insulin glargine dose was 44 U (0.5 U/kg)7
- The primary objective was to demonstrate noninferiority of Mounjaro 10 mg and/or 15 mg to insulin glargine in mean change from baseline in A1C at 52 weeks7
- The key secondary objectives were assessed at 52 weeks: noninferiority of Mounjaro 5 mg to insulin glargine in mean change from baseline in A1C and superiority of Mounjaro 5 mg, 10 mg, and/or 15 mg to insulin glargine in mean change from baseline in A1C, mean change from baseline in weight, and proportion of patients with A1C <7%7
- Study participants had a mean baseline A1C of 8.5% and a mean duration of T2D of 11.8 years7
Mounjaro vs placebo (+ insulin glargine ± metformin)
- SURPASS-5 was a 40-week, double-blind, placebo-controlled, phase 3 trial that randomized 475 adult patients with T2D who had inadequate glycemic control on insulin glargine 100 U/mL, with or without metformin (≥1500 mg/day), to receive once-weekly SC Mounjaro 5 mg, 10 mg, 15 mg, or placebo (1:1:1:1 ratio)1,8
- The background dose of insulin glargine was titrated to a fasting blood glucose of <100 mg/dL using a treat-to-target algorithm. The mean starting dose of insulin glargine was 34 U/day, 32 U/day, 35 U/day, and 33 U/day for patients on Mounjaro 5 mg, 10 mg, 15 mg, and placebo, respectively. At randomization, the initial insulin glargine dose in patients with A1C ≤8.0% was reduced by 20%. At 40 weeks, the mean dose of insulin glargine was 38 U/day, 36 U/day, 29 U/day, and 59 U/day for patients on Mounjaro 5 mg, 10 mg, 15 mg, and placebo, respectively1
- The primary objective was to demonstrate superiority of Mounjaro 10 mg and/or 15 mg to placebo in mean change from baseline in A1C at 40 weeks8
- The key secondary objectives were assessed at 40 weeks: superiority of Mounjaro 5 mg to placebo in mean change from baseline in A1C, and superiority of Mounjaro to placebo in proportion of patients with A1C <7% (Mounjaro 5 mg, 10 mg, and 15 mg) and <5.7% (Mounjaro 10 mg and 15 mg), mean change from baseline in fasting serum glucose, and mean change from baseline in weight1,8
- Study participants had a mean baseline A1C of 8.3% and mean T2D duration of 13.3 years1,8
QD=once daily; QW=once weekly.
See the composite endpoint that measured A1C, weight, and hypoglycemia
References:
- Mounjaro. Prescribing Information. Lilly USA, LLC.
- American Diabetes Association. Professional Practice Committee. 6. Glycemic goals and hypoglycemia: Standards of care in diabetes—2024. Diabetes Care. 2024;47(suppl 1):S111-S125.
- Frías JP, Davies MJ, Rosenstock J, et al.; for the SURPASS-2 Investigators. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503-515.
- Data on File. Lilly USA, LLC. DOF-TR-US-0003.
- Rosenstock J, Wysham C, Frías JP, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial Lancet. 2021;398(10295):143-155.
- Ludvik B, Giorgino F, Jódar E, et al. Once-weekly tirzepatide versus once-daily insulin degludec as add-on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS-3): a randomised, open-label, parallel-group, phase 3 trial. Lancet. 2021;398(10300):583-598.
- Del Prato S, Kahn SE, Pavo I, et al.; for the SUPRASS-4 Investigators. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS-4): a randomised, open-label, parallel-group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811-1824.
- Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS-5 randomized clinical trial. JAMA. 2022;327(6):534-545.
- Data on File. Lilly USA, LLC. DOF-TR-US-0007.
- Data on File. Lilly USA, LLC. DOF-TR-US-0014.
- Data on File. Lilly USA, LLC. DOF-TR-US-0048.
- Kwan A, Maldonado JM, Wang H, Rasouli N, Wilding J. 719-P: Tirzepatide induces weight loss in patients with type 2 diabetes regardless of baseline BMI: a post hoc analysis of SURPASS-1 through -5 studies. Diabetes. 2022;71(suppl 1):719-P.
- Data on File. Lilly USA, LLC. DOF-TR-US-0031.